elisa test vs pcr|drugs detected by elisa technique : store The FeLV Quant RealPCR Test is available in combination with the IDEXX FeLV Antigen by ELISA or as a stand-alone PCR test. The FeLV Antigen by ELISA with FeLV Quant RealPCR Test, test code 26355 (or add-on test code 263551 when ordered with a CBC/chemistry profile), is recommended as a confirmatory and staging test following an in-clinic Sterilization by steam is carried out by a specialized pressure-induced chamber called an autoclave that uses high-pressure steam to sterilize equipment and supplies. It is one of the most common and the oldest .
{plog:ftitle_list}
The effectiveness of autoclaves in sterilising equipment and industrial applications is deeply rooted in their ability to destroy all microorganisms, including bacteria, viruses, spores, .
Generally speaking, antibody-based allergen tests fall into two categories: LFDs (lateral flow devices) and ELISA (enzyme-linked immunosorbent assay) tests. Both types of test have a place in hazard control—many companies use both in their quality control plans. Discover 35 key distinctions between PCR and ELISA assays in lab testing, exploring their methodologies, applications, and diagnostic accuracies. There are currently two primary types of COVID-19 tests being used to test patients for COVID-19: molecular tests (also known as nucleic acid, RNA or PCR tests) and rapid antigen tests.The FeLV Quant RealPCR Test is available in combination with the IDEXX FeLV Antigen by ELISA or as a stand-alone PCR test. The FeLV Antigen by ELISA with FeLV Quant RealPCR Test, test code 26355 (or add-on test code 263551 when ordered with a CBC/chemistry profile), is recommended as a confirmatory and staging test following an in-clinic
PCR vs ELISA In-Process Test Selection for the Food Industry Introduction Optimal in-process food safety practices, from raw materials testing through final product release, are critical factors toward the protection of public health. Therefore, understanding the . Stimulation of cells/tissue with a given stimulus (e.g., a cytokine) is a common experimental setup in any cell biology lab. The cellular response to the external stimulus e.g., the activation/deactivation of intracellular signaling pathways and/or the secretion of proteins is often the research goal, and there are a number of different methods that you can use to analyze .
This test has a specificity of 99%, 14,15 and its specificity is further increased when used in conjunction with Western blot (99.5%). 15 The advantage of the C6 antibody test is that it is more sensitive than 2-tier test ing during early infection (sensitivity 29%– 74% vs 17%–40% in early localized infection, and 56%–90% vs 27%–78% in .Detection of SARS-CoV-2 by antigen ELISA test is highly swayed by viral load and sample storage condition. Nihad Adnan. . For GoTaq 1-Step RT-qPCR System, the RT-PCR thermal cycling initiated with reverse transcription at 50°C for 30 minutes followed by hot-start activation at 95°C for 2 minutes, 45 cycles of denaturation at 95°C for 15 . Thirteen studies examined a two-tier serological test protocol vs. clinical diagnosis, 24 studies examined single assays vs. clinical diagnosis, 9 studies examined single immunoblot vs. clinical diagnosis, 7 studies compared culture or PCR direct detection methods vs. clinical diagnosis, 22 studies compared two or more tests with each other and . Referral laboratories also conduct enzyme-linked immunosorbent assay (ELISA) as a screening test, as well as antibody-based Western blotting (targeting more antigens than the in-clinic test targets) and polymerase chain reaction (PCR) testing as follow-up tests to detect viral proviral DNA, viral DNA, or both. Point-of-Care Test Kits for FIV
Testing an individual animal There are very reliable tests available to determine which animals are infected. The ELISA test is reported to be 95% sensitive for animals infected 55 days. Any animal that develops antibodies is considered infected, because animals exposed to the virus and developing antibodies, but not remaining infected, have not been demonstrated.
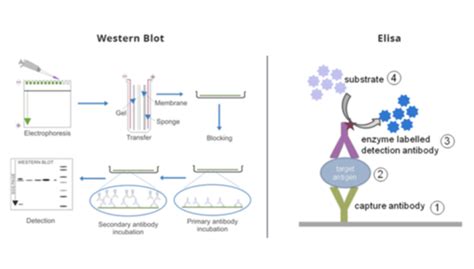
pcr vs western blot
ELISA-Test vs. PCR: In diesem Blog Artikel erörtern wir wo die Gemeinsamkeiten und Unterschiede zwischen einem ELISA und einem PCR-Test liegt. Im allgemeinen werden Diagnoseverfahren in der Medizin für die Identifizierung und Behandlung von Krankheiten genutzt. Ob aus der Schule, dem Studium oder dem Labor, sicherlich ist vielen der ELISA .Test: Enzyme-linked immunosorbent assay (ELISA) for the glutamate dehydrogenase (GDH) antigen What it tests for: The presence of C difficile organisms Commonly known as the antigen test, this test uses antibodies to test for presence of the GDH enzyme, a protein preserved in all C difficile bacteria. It is an excellent screening test because it has sensitivity greater than 90%, a . ELISA vs. Western Blot. ELISA and western blot are two popular methods for detecting and analyzing proteins. While ELISA is an exceptional tool for protein detection and quantification, the western blot is often used to confirm the result of an ELISA test as it is more likely to provide a definite result. It can also obtain extensive . Background Most current guidelines recommend two serological tests to diagnose chronic Chagas disease. When serological tests are persistently inconclusive, some guidelines recommend molecular tests. The aim of this investigation was to review chronic Chagas disease diagnosis literature and to summarize results of ELISA and PCR performance. Methods A .
Enzyme immunoassays (EIAs) use the catalytic properties of enzymes to detect and quantify immunologic reactions. Enzyme-linked immunosorbent assay (ELISA) is a heterogeneous EIA technique used in clinical analyses.[1] In this type of assay, one of the reaction components is nonspecifically adsorbed or covalently bound to the surface of a solid .
Commercially available enzyme-linked immunosorbent assay (ELISA) tests show potential for SARS-coronavirus-2 (SARS-CoV-2) diagnosis, especially in patients who have had symptoms for ≥11 days, according to the results of a study recently published in the Journal of Infectious Diseases.. Polymerase chain reaction (PCR)-based tests have become the .
Another technique worth mentioning is the combination of ELISA with PCR, known as PCR-ELISA. This method enables direct quantification of DNA immobilized on a solid surface through PCR. However, since this article primarily focuses on the analysis method of ELISA, it will not discuss PCR-ELISA. ELISA Applications:
elisa vs pcr difference
elisa result interpretation
elisa immunoassay technique
ELISA test is better than a Rapid or an RT-PCR test. The RT-PCR, ELISA, and RAPID tests are various diagnostic methods used to detect pathogens or antibodies to the pathogen based on the disease life cycle. The Three Techniques have their own unique sets of advantages and disadvantages, and hence it is vital to make the right choice for . PCR-ELISA, on the other hand, is in principle capable of detecting genomic material from any biological specimen, as long as suitable primers can be designed to amplify the target DNA sequence. . PCR amplicons from test samples are incubated with with biotinylated probes under conditions that allow sequence-specific hybridization of DNA .
Introduction IgG antibodies against T. gondii persist for years, and can act as a reliable serological biomarker for the diagnosis of previous exposure to this parasite. Hence, the current investigation was designed to compare diagnostic power of immuno-polymerase chain reaction (iPCR) and enzyme-linked immunosorbent assay (ELISA) methods for detection of T. .
The presence of HBs-Ag in the serum of patients was measured using the immunochromatographic and ELISA methods, and the specificity and sensitivity of those procedures were compared with the specificity and sensitivity of the PCR method. The immunochromatographic test had considerably greater sensitivity than the ELISA test, with .
Two-tiered Lyme disease testing uses two tests. The first is a screening test that should detect anyone who might have the disease. Tests that do this well have are regarded as having high sensitivity. This test is followed by a second test that is intended to make sure that only people with the disease are diagnosed. The RT-PCR test has so far been described as the most reliable test, but it only detects the presence of the virus. In other words, it detects the virus from the moment of infection, throughout the course of the disease (whether or not symptoms appear) and until the immune system is able to fight and eliminate the virus, and therefore it is no longer detectable.The versatility of PCR has led to the development of numerous specialized variants, such as quantitative PCR (qPCR), digital PCR (dPCR), and reverse transcription PCR (RT-PCR). NAAT techniques, including PCR, have expanded the application range even further.
Introduction: IgG antibodies against T. gondii persist for years, and can act as a reliable serological biomarker for the diagnosis of previous exposure to this parasite. Hence, the current investigation was designed to compare diagnostic power of immuno-polymerase chain reaction (iPCR) and enzyme-linked immunosorbent assay (ELISA) methods for detection of T. gondii .The sensitivity, specificity, and negative and positive predictive values of the first test were 77.5%, 99.3%, and 98.8% and 86.1%, respectively, taking ELISA as the standard test. Our study highlights that RDTs fare poorly compared to ELISA as screening assays and that reactive results by RDTs need to be confirmed by western blot for a . This means that serological detection is more effective than RT-PCR at the middle–late stage of infection. Moreover, serological detection results can reflect the severity of the patient’s symptoms, while RT-PCR results can hardly do. . Waleed established an ELISA test to detect antibodies against the SARS-CoV-2 S protein and S1/S2 .
The amplifying property of PCR allows the test to successfully detect even the smallest amount of coronavirus genetic material in a sample. This makes it a highly sensitive and accurate test. With accuracy that approaches 100%, it is the gold standard for diagnosing SARS–CoV–2. However, PCR tests have some weaknesses too.
7180 chemistry analyzer
mindray ba 88a semi-auto biochemistry analyzer
Discover practical tips to avoid wet packs in sterile processing, ensuring effective sterilization and maintaining the highest standards of patient safety.
elisa test vs pcr|drugs detected by elisa technique